What is the microscopic different between quartz Crystal and man-made crystal?
Names can sometimes be misleading. Some products called themself “crystal”. However, they are not the natural crystals you might imagine but rather than a types of glass. Both crystal and glass are primarily composed of silicon dioxide. But why are they different?
The answer is in the microscopic structure.

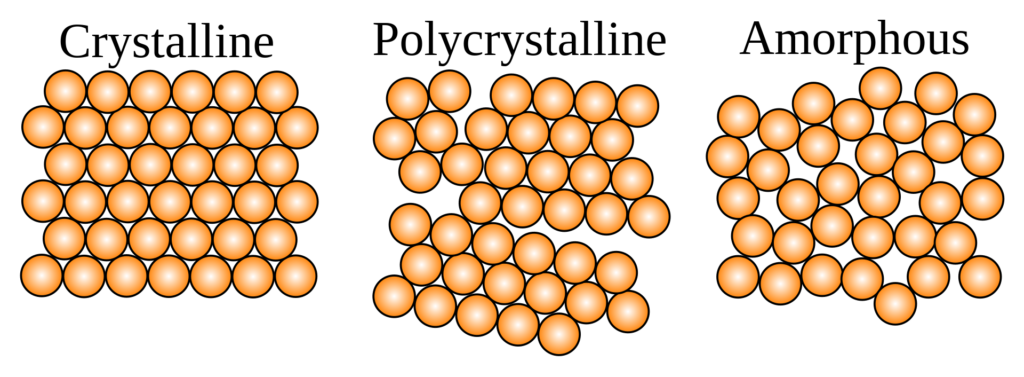
Glass belongs to the category of amorphous solids, in which atoms have no periodic structure whatsoever. Wax and many plastics are typical examples of amorphous solids.
Crystal or crystalline solids have atoms arranged in a near-perfect periodic microscopic arrangement. This category of solid material consists of constituent atoms, ions, or molecules arranged in a highly ordered repeating pattern known as a crystal lattice. This regular arrangement gives crystalline solids their characteristic properties, such as well-defined faces and angles, and is responsible for their ability to form distinct crystal shapes. Natural quartz, diamond, and table salt are examples of large crystals.
This is why glass can only be melted into various forms, while natural crystals possess geometric structures so fascinating that glass cannot achieve.
